Overview of the Ryan Lab
The Ryan Lab strives to answer fundamental questions in neuroscience, develop new tools for collaborative investigation into the mechanisms underlying neurological disorders and train scientists in the areas of molecular neuroscience and stem cell biology.
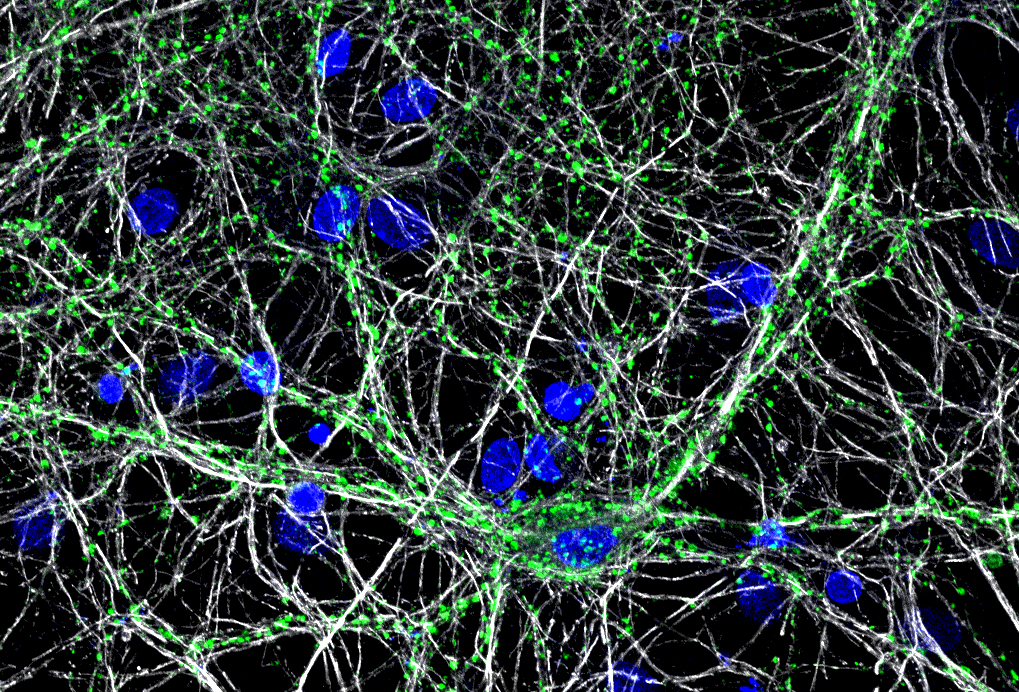
VGAT vesicular transporter in human induced pluriopotent stem cell derived neurons
When studying the mechanisms of neurodegenerative disease, the traditional animal systems, while powerful, have limitations to what they can teach. These systems utilize genetic over-expression or silencing paradigms to understand basic processes. While animal models have lead to huge advancements in our understanding of neurobiology, there is controversy over whether overexpression/silencing of gene expression is representative of diverse disease states. Indeed, the lack of availability of primary human neurons has made evaluating the pathological consequences of genomic mutations arduous. The use of human induced pluripotent stem cell (hiPSC) technology overcomes these limitations by providing a source of human neurons from both normal and disease genetic backgrounds. We currently focus on stem cell based models of Parkinson's Disease (PD) to study how mitochondrial stress mechanisms impact on neuronal function in human disease. To learn more, click the link above.
Nature (2010) 465, 704-712 Nature
Learning and memory has at its core modifications to tiny spine like structures that exist on a special subset of neurons in the brain known as “spiny neurons.” These spines must move, adapt, grow and retract in response to chemical neurotransmitters in the brain, a process that as a whole is believed to be the cellular manifestation of learning, known as synaptic plasticity. The spines themselves are thought to serve as basic units of memory storage. While much work has focused directly on the chemical signals that mediate communication in the brain and the ion channels that are opened as a result, little is known of the specific signalling pathways that control the actual generation, shape and loss of individual spines. Nitric Oxide is unique second messenger in that it can directly alter the proteins responsible for dynamic changes to spine morphology. Nitric Oxide signalling may regulate synaptic plasticity by altering the density and morphology of spines through a process called S-nitrosylation. We are monitoring what proteins are S-nitrosylated by Nitric Oxide under conditions of spine growth, spine stabilization and spine retraction. This research will further our understanding neural architecture and the means by which cells of the brain communicate. To learn more, click the link above.
GFP expressing neurons allow for visualization of dendritic spines within complex neuronal networks
Stem cells are found throughout the body during both development and adulthood. They have the unique capacity to continuously divide and to produce daughter cells with a multitude of identities. In the nervous system, this process give rise to neural precursor cells that populate the developing brain with both neurons and glia, while renewing their own population at the same time. We are attempting to overcome the limited repair observed in many neurological disorders by employing directed differentiation to replenish lost or damaged tissue in the brain. To learn more, click the link above.
Schematic diagrams show published protocols for the generation of midbrain dopamine (mDA) neurons for the potential treatment of Parkinson's disease, striatal neurons for the treatment of Huntington's disease and glial precursors for the treatment of demyelinating disorders. Small molecules and growth factors that are used to direct cell fate are indicated below the arrows; the factors that are induced or inhibited are shown in parentheses. Nature Reviews Genetics (2014) 15:82–92